Wholesale Clean Room Door - IV Infusion Glass Bottle Turnkey Project – IVEN
Wholesale Clean Room Door - IV Infusion Glass Bottle Turnkey Project – IVEN Detail:
Product Description:
IVEN Pharmatech is the pioneer supplier of turnkey plants that provides integrated engineering solution for worldwide pharmaceutical factory such as IV solution, vaccine, oncology etc., in compliance with EU GMP, US FDA cGMP, PICS, and WHO GMP.
We provide the most reasonable project design, the high quality equipment and the customized service to different pharmaceutical and medical factories from A to Z for Non-PVC soft bag IV solution, PP bottle IV solution, Glass vial IV solution, Injectable Vial & Ampoule, Syrup, Tablets & Capsules, Vacuum blood collection tube etc.

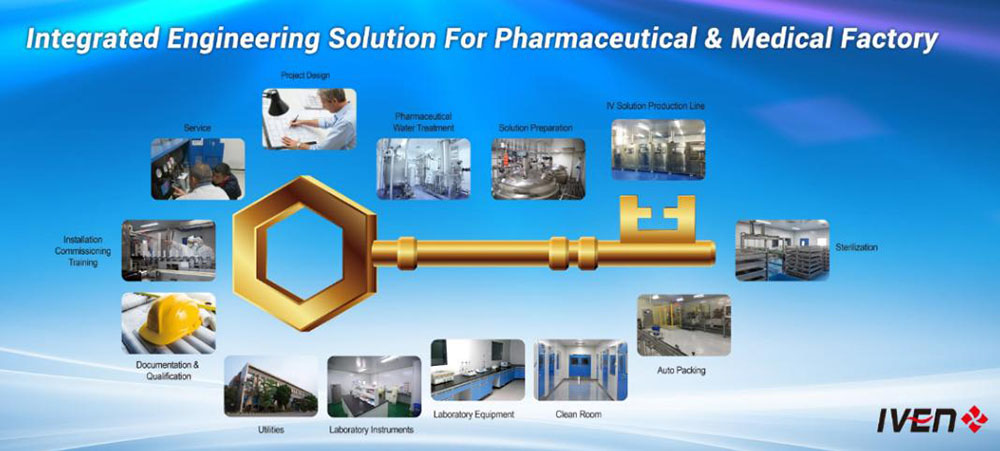

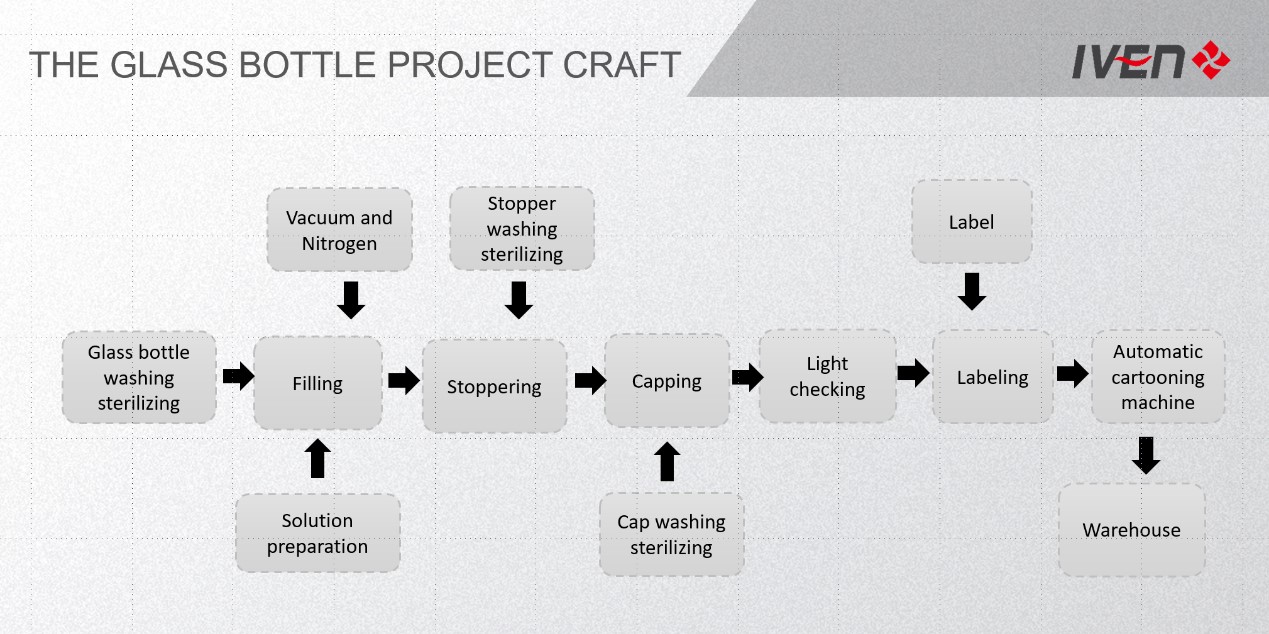
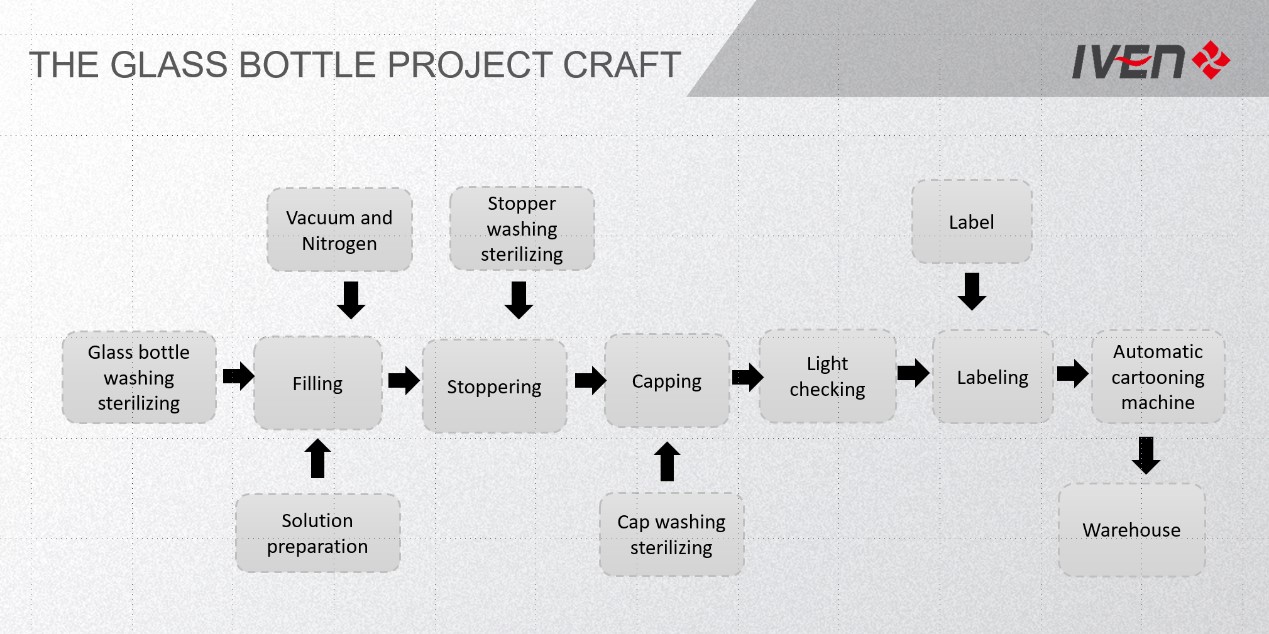
Core description
SHANGHAI IVEN PHAMATECH is considered as leader for IV solution turnkey projects supplier.
In our turnkey offers we usually exclude those items that can be procured locally at reasonable prices by the customer itself (like land, buildings, brick wall parts…).
IVEN besides can provide the Turnkey project also can help client for below work:
– Advanced Know How Transfer for additional Glass bottle IV solutions.
– Post-Start up assistance
– Raw Materials and Consumables
– Black Utilities
STANDARD IV SOLUTION PRODUCTS &TPN
- NaCl 0,18 – 2.7%
- Glucose 2,5 – 50%
- Sodium Lactate (Hartmanns’s) Solution
- Ringer Lactate
- Water For injection
- Sterile Water for Irrigation
- Sodium Chloride 0.9% for irrigation
- Sodium Chloride
- Potassium Chloride 0.15 – 0.3% in Sodium Chloride 0.9%
- Potassium Chloride 0.15 – 0.3% in Glucose 5%
TPN (Total Parenteral Nutrition)
- Amino acids
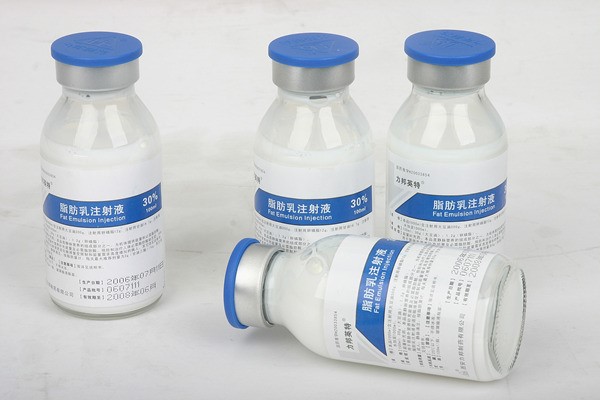
- Fat Emulsion
SPECIAL SOLUTIONS
- Paracetamol
- Plasma Expanders
- Mannitol
- Lidocainje hydrochloride 0.4% and glucose 5%
- Sodium bicarbonate 1.26 – 4.2%
- Phosphate IV solution
- Metronidazole
- Ciprofloxacin
- Levofloxacin
- Fluconazol
FROM DESIGN TO VALIDATION, WE ARE ALWAYS CONSIDERATION FOR OUR CLIENT.
The standards of good manufacturing practice (cGMP) require special attention to risk assessment and verification procedures: “… it is requirement of good manufacturing identify the activities of validation necessary to demonstrate control critical aspects of particular operations. The significant changes made to installations, equipment and processes, which may affect product quality, should be validated. A procedure for risk assessment should be used to determine the scope and extent of validation.” The Validation Master Plan serves to make sure that all equipment, procedures, that may affect the quality or integrity or effectiveness of the product, are validated; it contains the general principles which comply during the validation task, and plans activities to be carried out for this purpose.





IVEN TURNKEY FOR IV FLUIDS AND PARENTERAL SOLUTIONS / STANDARD STEPS
√ Basic Engineering √ Detailed Engineering √ Design Qualification √ Inlet Water Pretreatment Plant √ Pharmaceutical Water Systems (Softened, Purified and Distilled Water) √ Pharmaceutical Processing and Solution Preparation Systems √ Pharmaceutical Bottle washing, Filling, Inspecting, Packaging lines √ Clean Rooms √ Epoxy coating of the floors/PVC floor √ HVAC and air treatment plant √ Autoclave √ Pure Steam Generator and PS circuit √ Laboratories of Analysis (Microbiological / Chemical) √ Site Master Plan √ Validation Master Plan √ Installation √ Training √ Start up √ Technical Files & Documentation √ IQ/OQ √ PQ Protocols √ Validation at Site √ Standard Operating Procedures √ Initial Know How Transfer √ GMP preAudit √ Spareparts for n years
IVEN Oversea Turnkey Projects
Till now, we’ve already provided hundreds sets of pharmaceutical equipment and medical equipment to more than 40 countries. Meanwhile, we helped our customers to built the pharmaceutical and medical plant with turnkey projects in Russia, Uzbekistan, Tajikistan, Indonesia, Thailand, Saudi, Iraq, Nigeria, Uganda etc. All these projects won our customers and their government high comments.
IVEN profession and experience can help you finish whole IV solution turnkey plant in the shortest time and avoid all kinds of potential risks:






IVEN oversea pharmaceutical turnkey plants customers:


Meanwhile, we helped our customers to built 20+ pharmaceutical and medical turnkey plants in Russia, Uzbekistan, Tajikistan, Indonesia, Thailand, Saudi, Iraq, Nigeria, Uganda, Tanzania, Ethiopia, Myanmar etc, mainly for IV solution, injectable vials and ampoules. All these projects won our customers and their government high comments.
We also exported our IV solution production line to Germany.


Indonesia IV bottle turnkey plant
Vietnam IV bottle turnkey plant


Uzbekistan IV bottle turnkey plant

Thailand Injectable vial turnkey plant
Tajikistan IV bottle turnkey plant

Saudi Arabia IV bag turnkey plant
Product detail pictures:
Related Product Guide:
Our enterprise since its inception, usually regards product top quality as business life, repeatedly enhance manufacturing technology, make improvements to product excellent and continuously strengthen enterprise total high quality administration, in strict accordance with all the national standard ISO 9001:2000 for Wholesale Clean Room Door - IV Infusion Glass Bottle Turnkey Project – IVEN , The product will supply to all over the world, such as: Vietnam, Philippines, Slovenia, Our well-equipped facilities and excellent quality control throughout all stages of production enable us to guarantee total customer satisfaction. If you are interested in any of our products or would like to discuss a custom order, please feel free to contact me. We are looking forward to forming successful business relationship with new clients around the world.
Good quality and fast delivery, it's very nice. Some products have a little bit problem, but the supplier replaced timely, overall, we are satisfied.