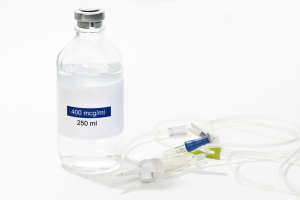
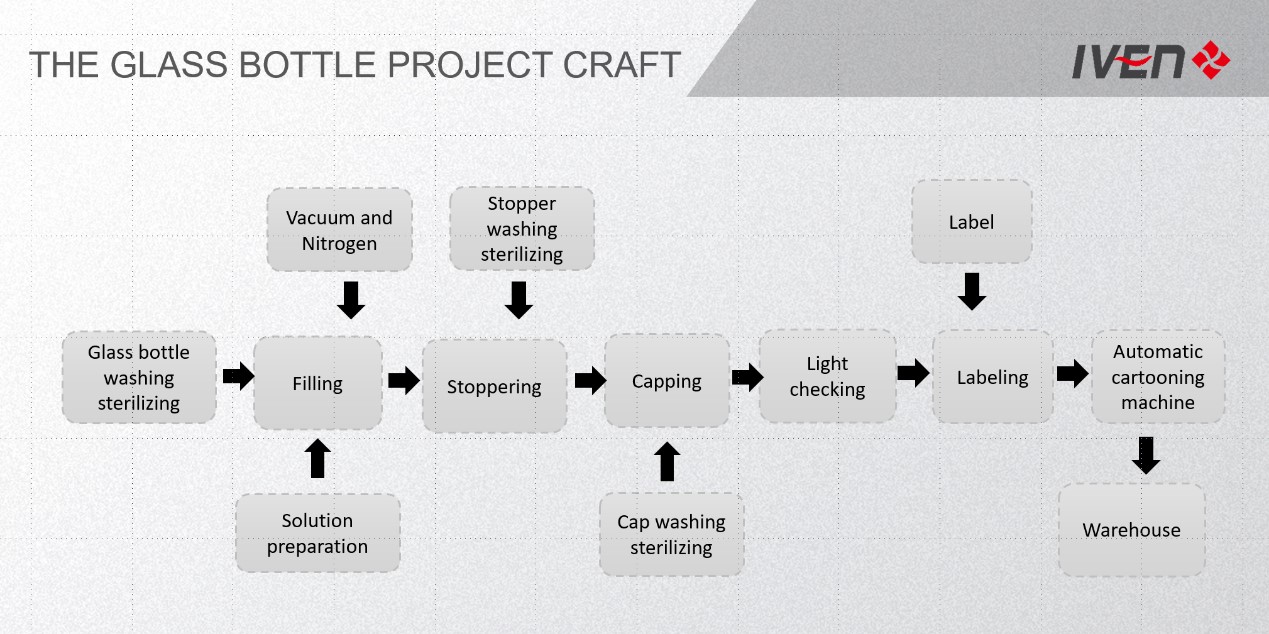
IV Infusion Glass Bottle Turnkey Project
IVEN Pharmatech is the pioneer supplier of turnkey plants that provides integrated engineering solution for worldwide pharmaceutical factory such as IV solution, vaccine, oncology etc., in compliance with EU GMP, US FDA cGMP, PICS, and WHO GMP.
We provide the most reasonable project design, the high quality equipment and the customized service to different pharmaceutical and medical factories from A to Z for Non-PVC soft bag IV solution, PP bottle IV solution, Glass vial IV solution, Injectable Vial & Ampoule, Syrup, Tablets & Capsules, Vacuum blood collection tube etc.

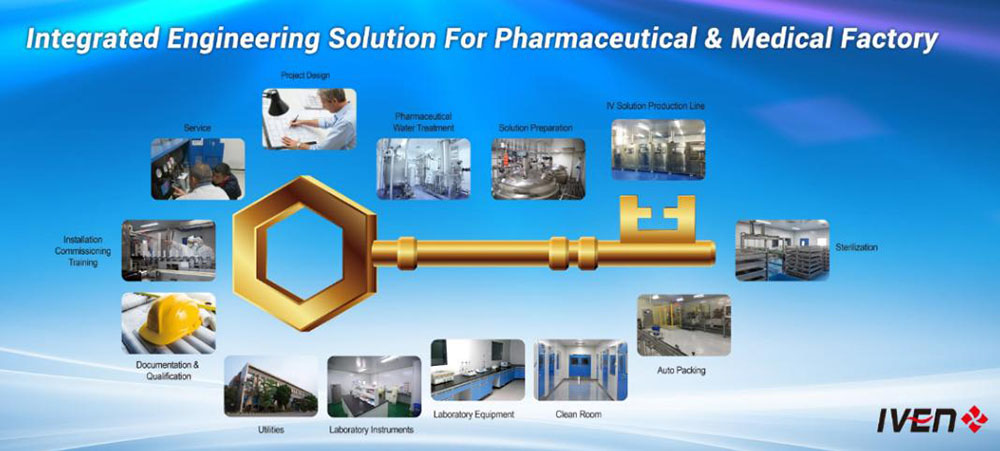

In our turnkey offers we usually exclude those items that can be procured locally at reasonable prices by the customer itself (like land, buildings, brick wall parts…).
IVEN besides can provide the Turnkey project also can help client for below work:





Till now, we’ve already provided hundreds sets of pharmaceutical equipment and medical equipment to more than 40 countries. Meanwhile, we helped our customers to built the pharmaceutical and medical plant with turnkey projects in Uzbekistan, Tajikistan, Indonesia, Thailand, Saudi, Iraq, Nigeria, Uganda etc. All these projects won our customers and their government high comments.






IVEN profession and experience can help you finish whole IV solution turnkey plant in the shortest time and avoid all kinds of potential risks:






IVEN have a very professional technical and engineering team, our onsite training and after-sales support can give long term technical assurance for your NON-PVC IV fluid turnkey plant:
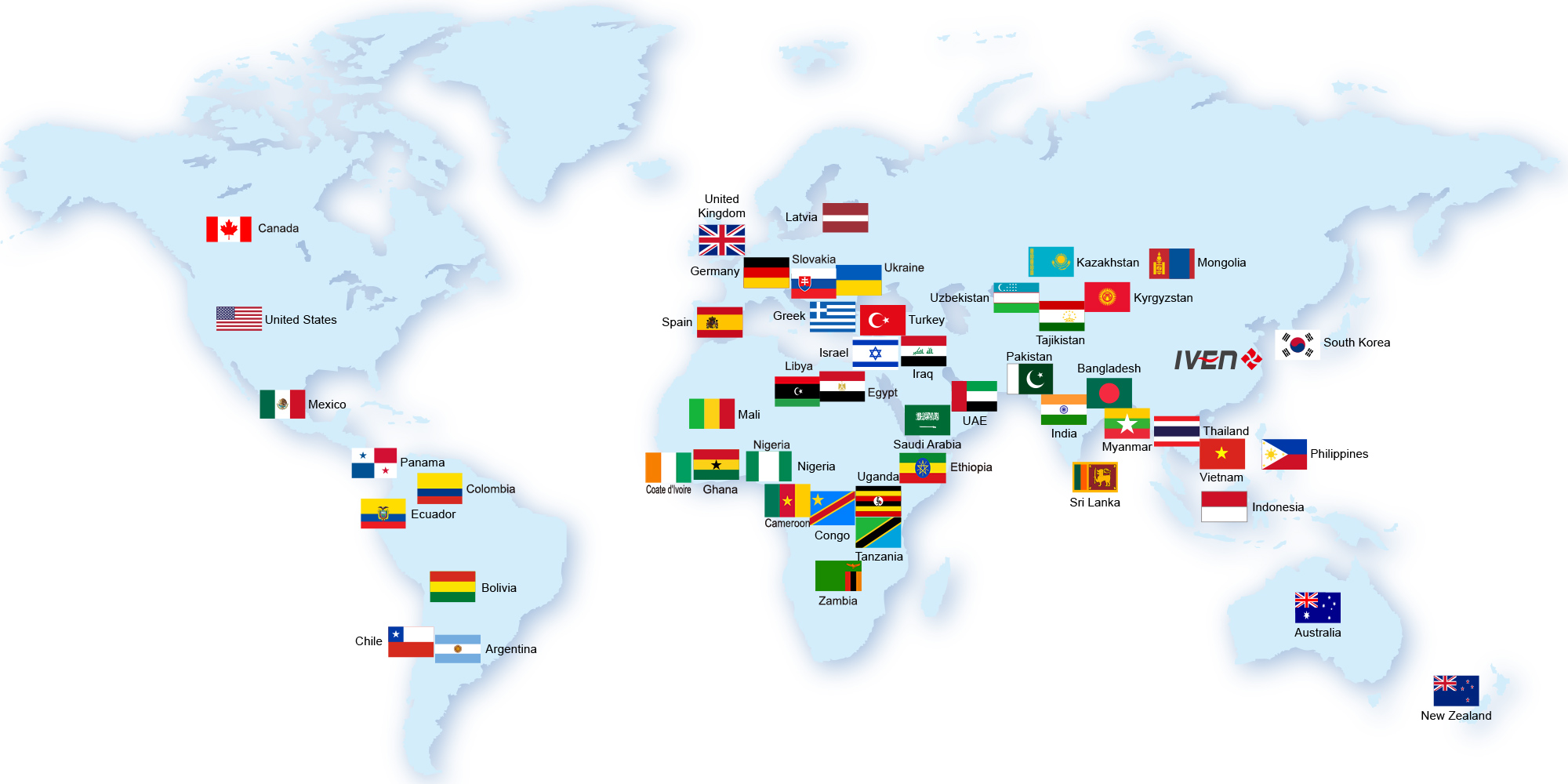
Till now, we’ve already provided hundreds sets of pharmaceutical equipment and medical equipment to more than 50 countries.
Meanwhile, we helped our customers to built 20+ pharmaceutical and medical turnkey plants in Uzbekistan, Tajikistan, Indonesia, Thailand, Saudi, Iraq, Nigeria, Uganda, Tanzania, Ethiopia, Myanmar etc, mainly for IV solution, injectable vials and ampoules. All these projects won our customers and their government high comments.
We also exported our IV solution production line to Germany.


Indonesia IV bottle turnkey plant
Vietnam IV bottle turnkey plant


Uzbekistan IV bottle turnkey plant

Thailand Injectable vial turnkey plant
Tajikistan IV bottle turnkey plant